 |
|
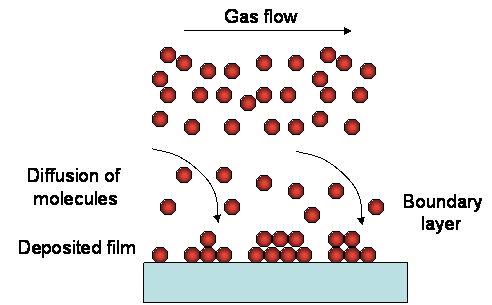
GAS FLOW in a reaction chamber
-
The gas flow is given not in volume per time, but in number per time, or concentration of molecules in the chamber
-
On average the flow will move from high to low pressure, to reduce the pressure gradient
-
The pressure gradient is created by introducing gas in a chamber and pumping out the gas (see picture below)
-
A part of the gas molecules will diffuse toward the substrate and react at the surface
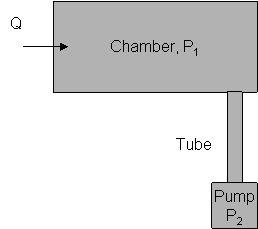
- Another parameter that characterises the vacuum system is volumetric flow or pumping speed
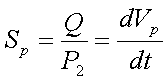
- it is defined as the variation of volume in time
- it is measured in standard cubic centimeters per minute (sccm) = the volume of gas at 1 atm and 273 K (standard conditions)
- it is equal to the throughput divided by pressure
- it is the most widely used parameter to define the flow passing through the reactor, at a certain pressure
- it defines also the pump capacity to maintain a certain pressure for a given gas flow
-
The conductance of a pipe (or a vacuum component) connecting the chamber to the pump (pipes reduces the capacity of pumping) is defined as: 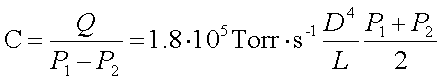 , therefore the pipes should be short and with a large diameter, so that the gas can be efficiently pumped -
Knudsen number gives information about the type of flow: 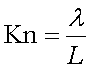 (defined as the ratio between mean free path and reactor's length)
-
Kn > 1 - Molecular flow (if the mean free path is larger than the reactor dimensions, the molecules do not collide at all before meeting the walls, and the gas behaves like a sum of molecules, instead of a fluid)
-
1 > Kn > 0.01 - Intermediate flow
-
0.01 > Kn - Viscuous flow (if the mean free path is much smaller than the reactor dimensions, a huge number of collisions take place and the gas behaves as a fluid) |
|
