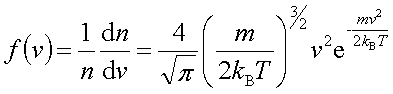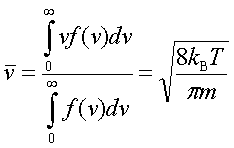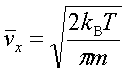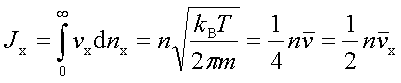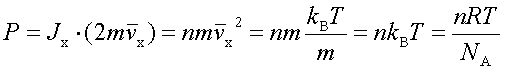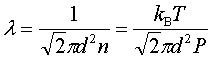|
|
||
|
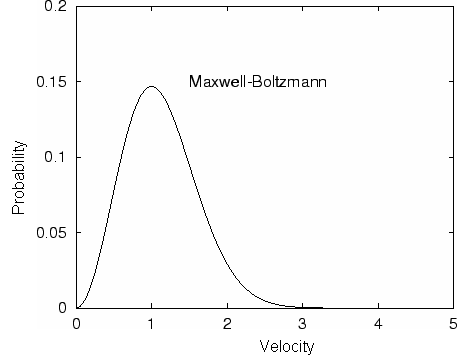
In processes such as deposition (CVD or PVD) or thermal growth, gases are introduced in the reactors. Thus a few notions of gas kinetics are necessary for a better understanding. More about gas flow dynamics can be found in the module "Principle of CVD". Futhermore, most of the processes, including diffusion and implantation take place in vacuum or low pressure. Therefore also some notions about vacuum technology are given in this module. GAS KINETICS In the processes with gases, the velocity of the gas particles is an important factor. However, the particles have not a constant speed, and it is impossible to find out the velocity of each particle in a gas. (There are around 1019 particles in a cm3 of gas). Maxwell-Boltzmann distribution (shown in the picture below) can be used to find average velocities. The probabilty of having a certain velocity can be defined as in the expression below, depending on the mass of the gas molecule (m) and temperature (T). The probabilty has a maximum for average velocities, and goes to zero for higher or lower velocities.
If we calculate how may atoms reach the substrate in a second, at a very low pressure of 10-6 Torr, by writing the concentration as a function of pressure, we obtain that one monolayer is deposited.
|
 Substrate preparation
Substrate preparation