DEAL - GROVE MODEL
-
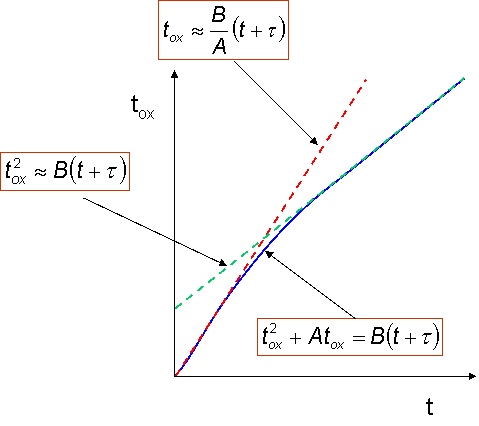
Shows how the oxide thickness varies in time as a function of oxidation conditions (pressure, temperature)
-
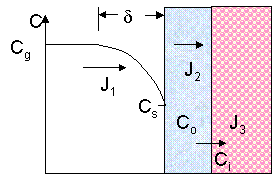
The flows of the oxidant gas is written as a function of concentrations in the three ambients (gas, oxide, silicon), in order to find out the oxide thickness. Notations: J1 is the flow of molecules in air arriving at the film surface, J2 is the flow of particles diffusing inside the oxide, J3 is the flow which reacts at the interface, Cg is the concentration of particles in the air, Co is the oxygen concentration in the oxide, Cs is the oxidant concentration at the surface and Ci is the concentration of molecules incorporated in the film. The reaction rate (R) is calculated from the equations written below.

|
|
