 |
|
- Reactive ion etching
- Anisotropic etch with higher selectivity than ion milling
- Selectivity is caused by the introduction of reactant gases - usually chlorine plasma
- Low pressure
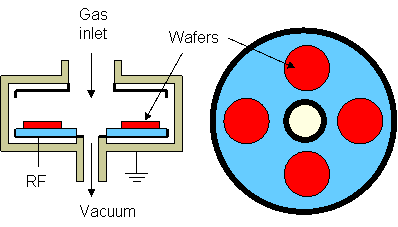
- Mechanism of RIE
- an undoped wafer is etched with avery low etch rate
- a heavily doped wafer is etched faster because:
- negatively charged Cl ions bond ionically to the surface, freeing additional chemisorbtion sites
- Cl penetrates the surface and creates volatile compounds
- Cl penetration is dramatically increased by ion bombardment, thus the etching is also anisotropic, not only selective
- Materials etched with RIE
- Al - formes a thin layer of Al2O3 in the air which is difficult to be removed with Cl plasma, thus it is removed in the beginning an Ar plasma for oxide removal
- Cu - is difficult to be etched with RIE because it formes compounds that are not volatile
- Photoresist can be attacked by Cl, thus it is necessary to use special photoresists for RIE
- GaAs - is etched in a hydrogen based + metane ambient, forming volatile AsH3
- Sidewall passivation can be also realised for increased selectivity
- Damage in RIE
- Substrate damage
- Chemical contamination - polymerization due to the photoresist, sputtering the chamber walls and redeposition on wafer, etc.
- the damage can be removed by O2 and H2 plasma treatments, at high anneal temperature
- Classification of plasma assisted etching
- In order to summarise the various etching methods described in this module, a graph was made of ion energy vs. pressure
- at low pressure, ions have high energies due to long mean free paths and the physical mechanism is present (higher anisotropy)
- at high pressure, ions have much lower energies, thus the etching is based more on the chemical gases introduced (higher selectivity)
- at intermediate pressures, ion assisted etching takes place in which both mechanisms are important
- depending on the goal, the preferable conditions can be chosen, for example if both selectivity and anisotropy are important, intermediate pressure can be the best solution
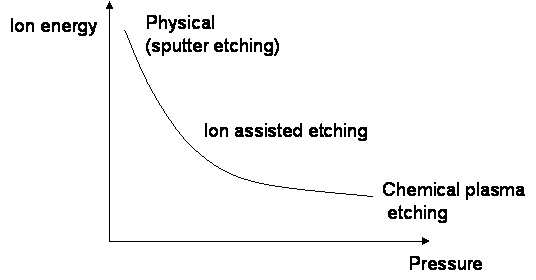 |
|
