|
|
||
|
Conductivity type
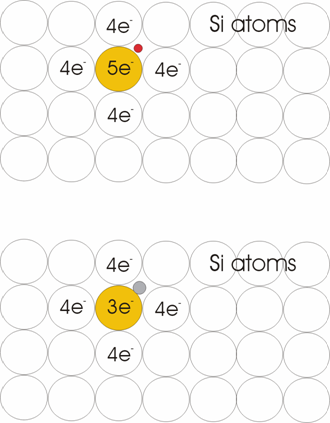
Resistivity Gives information on how strong is the opposition of the material to current flow. The substrate resistivity is modified by doping (introducing atoms with 5 or 3 electrons on the last layer). It is an important parameter, because other processes speed (e.g. oxidation) are influenced by the substrate resistivity. Normal value: 1-10 ohm per cm2.
|
 Substrate preparation
Substrate preparation